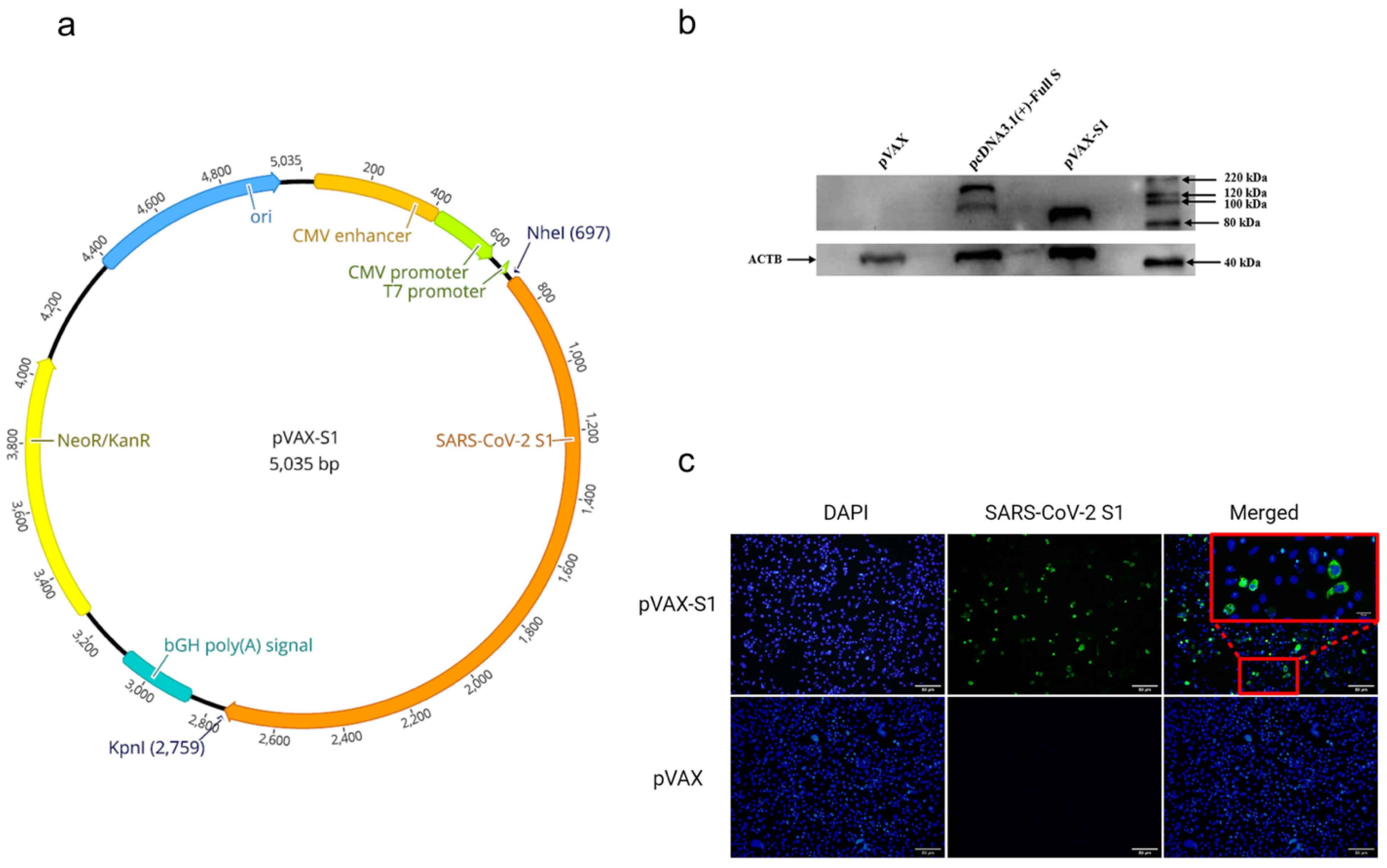
Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV
Por um escritor misterioso
Last updated 02 junho 2024

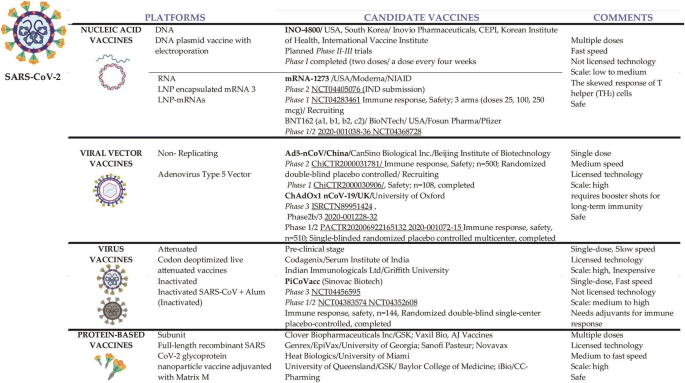
Recent advances, approaches and challenges in targeting pathways for potential COVID-19 vaccines development

704/DNA vaccines leverage cytoplasmic DNA stimulation to promote anti-HIV neutralizing antibody production in mice and strong immune response against alpha-fetoprotein in non-human primates: Molecular Therapy - Nucleic Acids

Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial - eClinicalMedicine

An Update on the Status of Vaccine Development for SARS-CoV-2 Including Variants. Practical Considerations for COVID-19 Special Populations - Bulent Kantarcioglu, Omer Iqbal, Joseph Lewis, Charles A. Carter, Meharvan Singh, Fabio Lievano
Vaccines, Free Full-Text
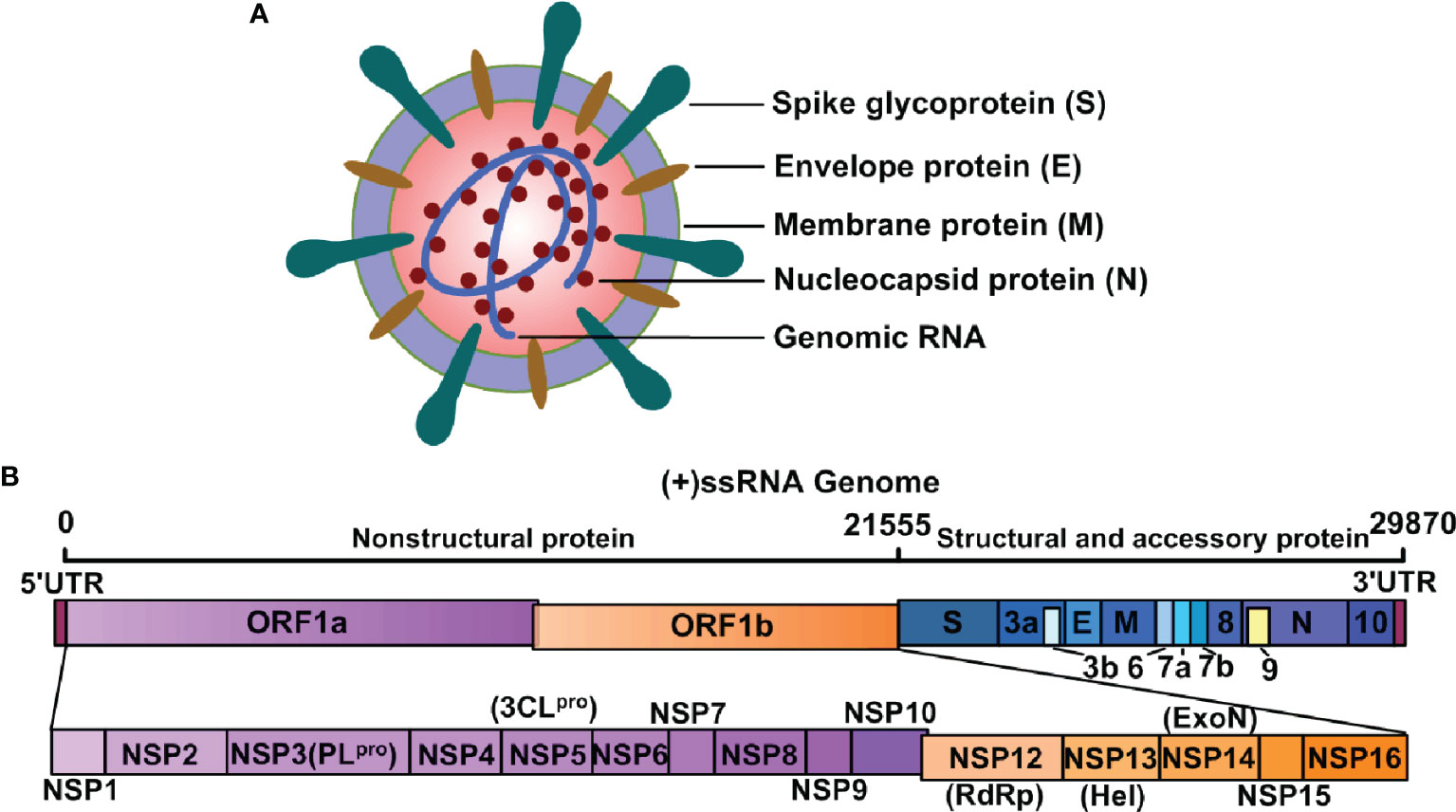
Frontiers Vaccines for COVID-19: A Systematic Review of Immunogenicity, Current Development, and Future Prospects

Current Update on Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine Development with a Special Emphasis on Gene Therapy Viral Vector Design and Construction for Vaccination
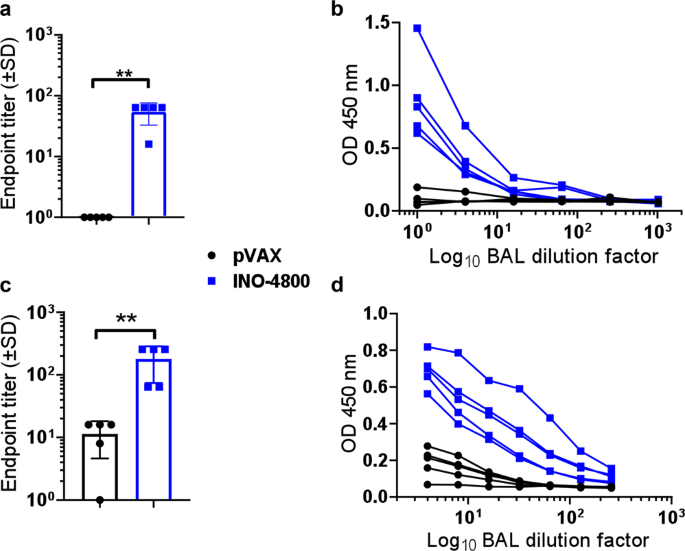
Immunogenicity of a DNA vaccine candidate for COVID-19

A brief review on DNA vaccines in the era of COVID-19
Recomendado para você
-
 Paid Newsletter 101: creation, pricing, examples, format ideas, tips02 junho 2024
Paid Newsletter 101: creation, pricing, examples, format ideas, tips02 junho 2024 -
 Household income in the United States - Wikipedia02 junho 2024
Household income in the United States - Wikipedia02 junho 2024 -
 The 9 Box Grid: How to Use It, Practical Template, And02 junho 2024
The 9 Box Grid: How to Use It, Practical Template, And02 junho 2024 -
 5 Best Life Coach Certification Programs Of 2023 – Forbes Health02 junho 2024
5 Best Life Coach Certification Programs Of 2023 – Forbes Health02 junho 2024 -
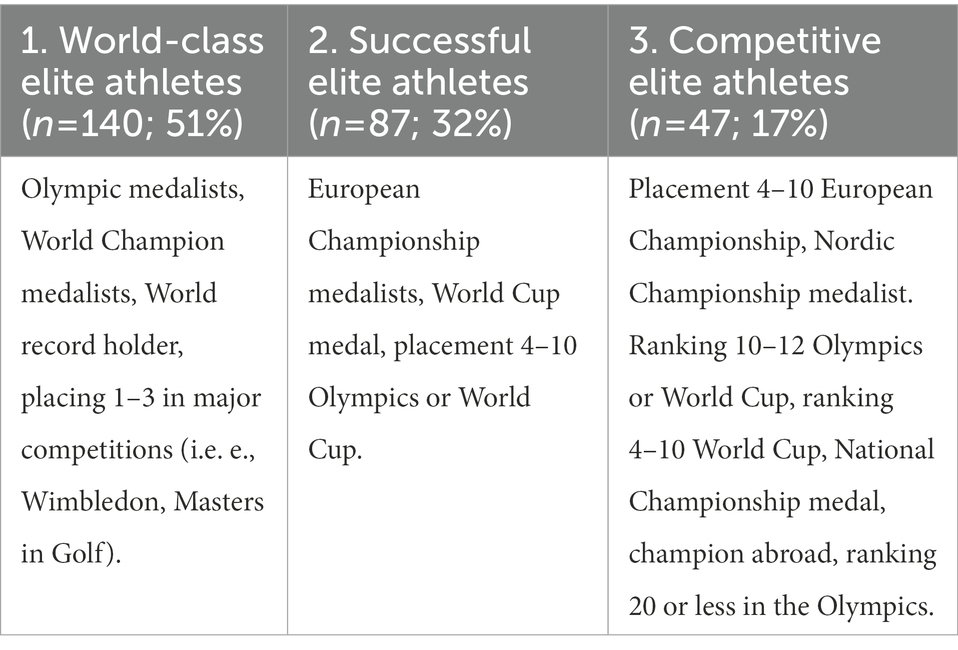 Frontiers Dual career support among world-class athletes in02 junho 2024
Frontiers Dual career support among world-class athletes in02 junho 2024 -
 2020 Digital transformation survey02 junho 2024
2020 Digital transformation survey02 junho 2024 -
 Home Care Software Designed By Nurses for Nurses- HCHB02 junho 2024
Home Care Software Designed By Nurses for Nurses- HCHB02 junho 2024 -
2023 Deloitte holiday retail survey02 junho 2024
-
 Accelerating policy response to curb non-communicable diseases: an02 junho 2024
Accelerating policy response to curb non-communicable diseases: an02 junho 2024 -
 The Ultimate Guide to Customer Education in SaaS: Best Practices02 junho 2024
The Ultimate Guide to Customer Education in SaaS: Best Practices02 junho 2024
você pode gostar
-
/i.s3.glbimg.com/v1/AUTH_08fbf48bc0524877943fe86e43087e7a/internal_photos/bs/2021/h/7/AXgy6PSRetK2fQ5WM3Ew/2014-03-14-playstation-3-ps3-ps-store-now-aluguel-catherine.jpg) Resumo da semana de notícias em jogos: Titanfall e RPG de Angry02 junho 2024
Resumo da semana de notícias em jogos: Titanfall e RPG de Angry02 junho 2024 -
 Legends of Runeterra: 40 Things You Need to Know About Riot's New Game - IGN02 junho 2024
Legends of Runeterra: 40 Things You Need to Know About Riot's New Game - IGN02 junho 2024 -
 As peripécias do anime ANOTHER02 junho 2024
As peripécias do anime ANOTHER02 junho 2024 -
 QUIZ DE MATEMÁTICA - 4º ANO - 5º ANO - SUBTRAÇÃO02 junho 2024
QUIZ DE MATEMÁTICA - 4º ANO - 5º ANO - SUBTRAÇÃO02 junho 2024 -
Alphabet lore x y02 junho 2024
-
 Homenagem a Gaby Leca (r) - ibisPaint02 junho 2024
Homenagem a Gaby Leca (r) - ibisPaint02 junho 2024 -
 PC: 10 jogos de terror para jogar de noite (se conseguir)02 junho 2024
PC: 10 jogos de terror para jogar de noite (se conseguir)02 junho 2024 -
 roupas para gacha Life02 junho 2024
roupas para gacha Life02 junho 2024 -
 Goddess Café Terrace Chapter 17: Akane's Circumstances - Novel Cool - Best online light novel reading website02 junho 2024
Goddess Café Terrace Chapter 17: Akane's Circumstances - Novel Cool - Best online light novel reading website02 junho 2024 -
 Why BIG brands are BIG and small brands are small02 junho 2024
Why BIG brands are BIG and small brands are small02 junho 2024