FDA won't comment on status of Emergency Use Authorizations for two antibody treatments
Por um escritor misterioso
Last updated 03 junho 2024

The US Food and Drug Administration told CNN Thursday morning that the agency doesn’t have any comments on the applications for Emergency Use Authorizations for Eli Lilly and Regeneron antibody treatments.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.

Eli Lilly's Antibody Treatment Gets Emergency F.D.A. Approval

Federal Register :: Authorizations of Emergency Use of Certain
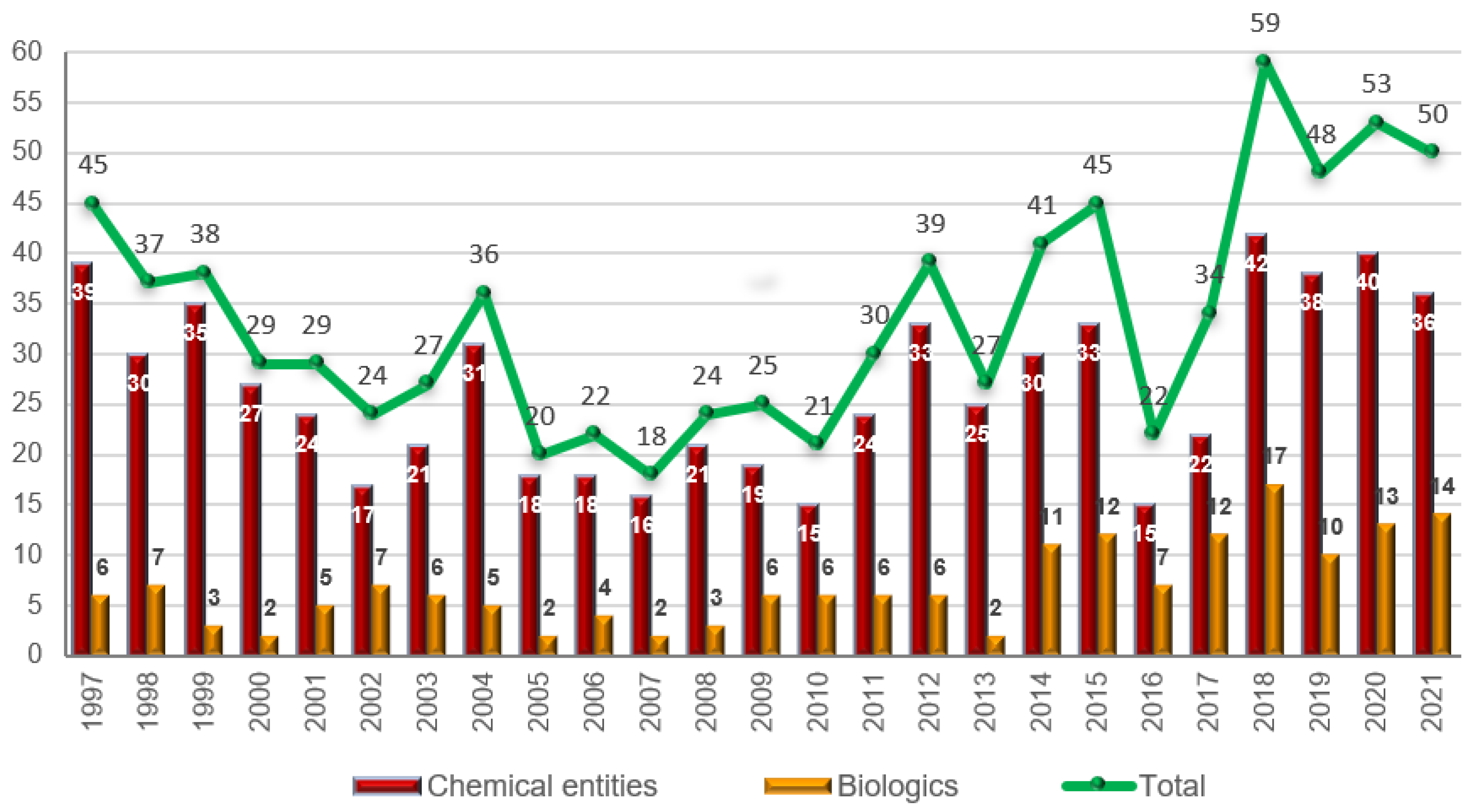
Molecules, Free Full-Text

FDA Advisory Committee Votes to Recommend Pfizer COVID-19 Vaccine

Federal Register :: Authorizations of Emergency Use of Certain
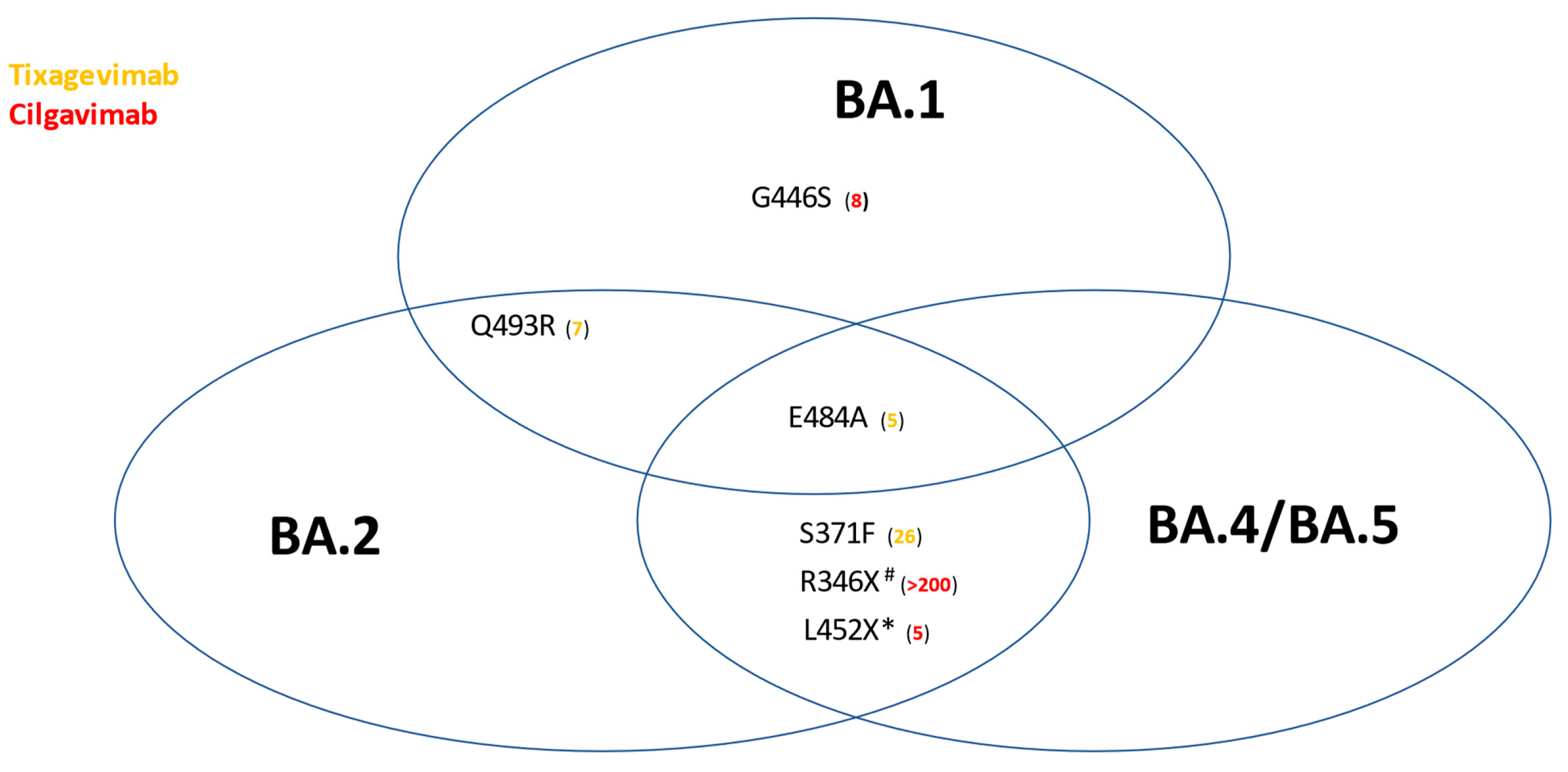
Viruses, Free Full-Text
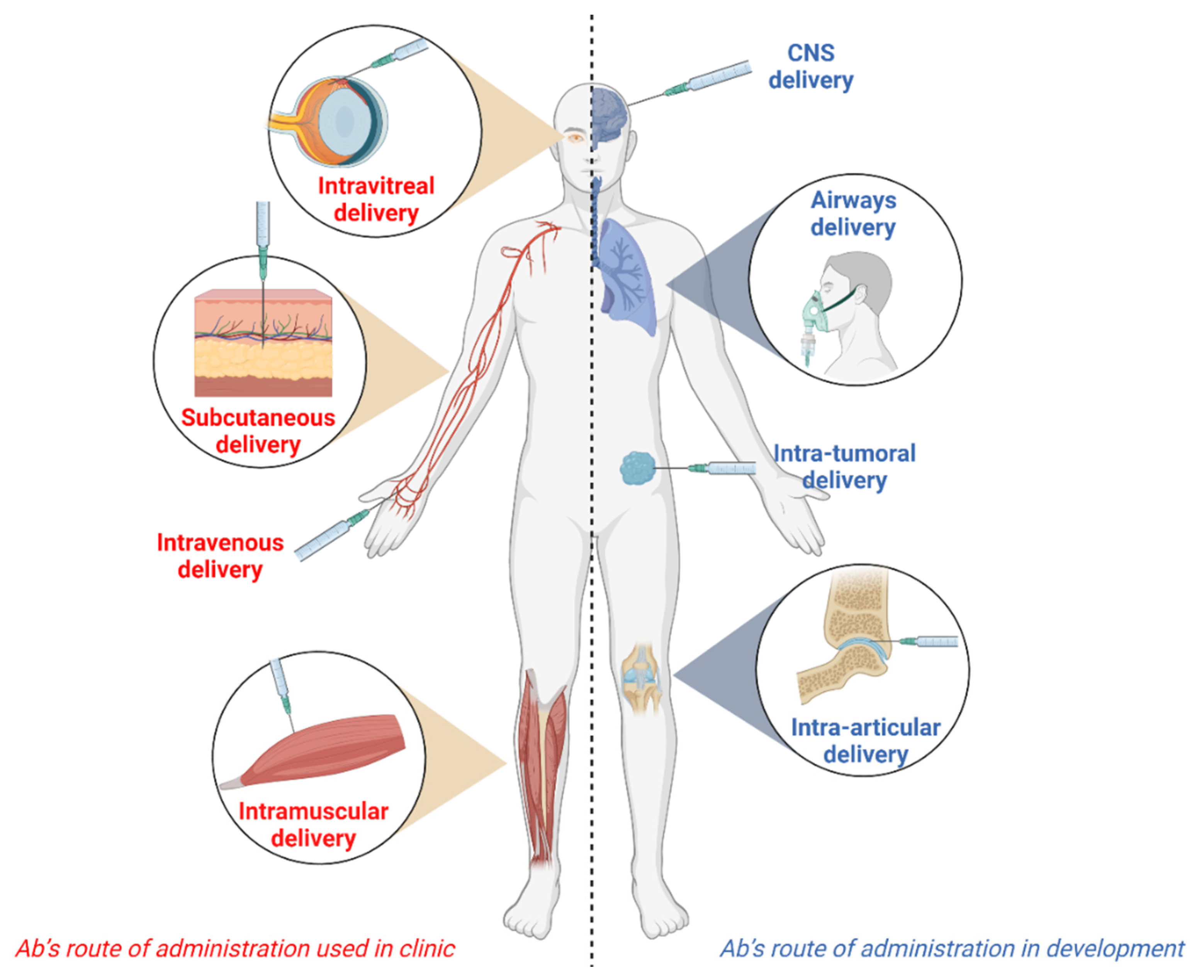
Antibodies, Free Full-Text

Experts recommend Pfizer's COVID-19 vaccine to FDA for emergency

Emergency Use Authorization Vs. Full FDA Approval: What's the
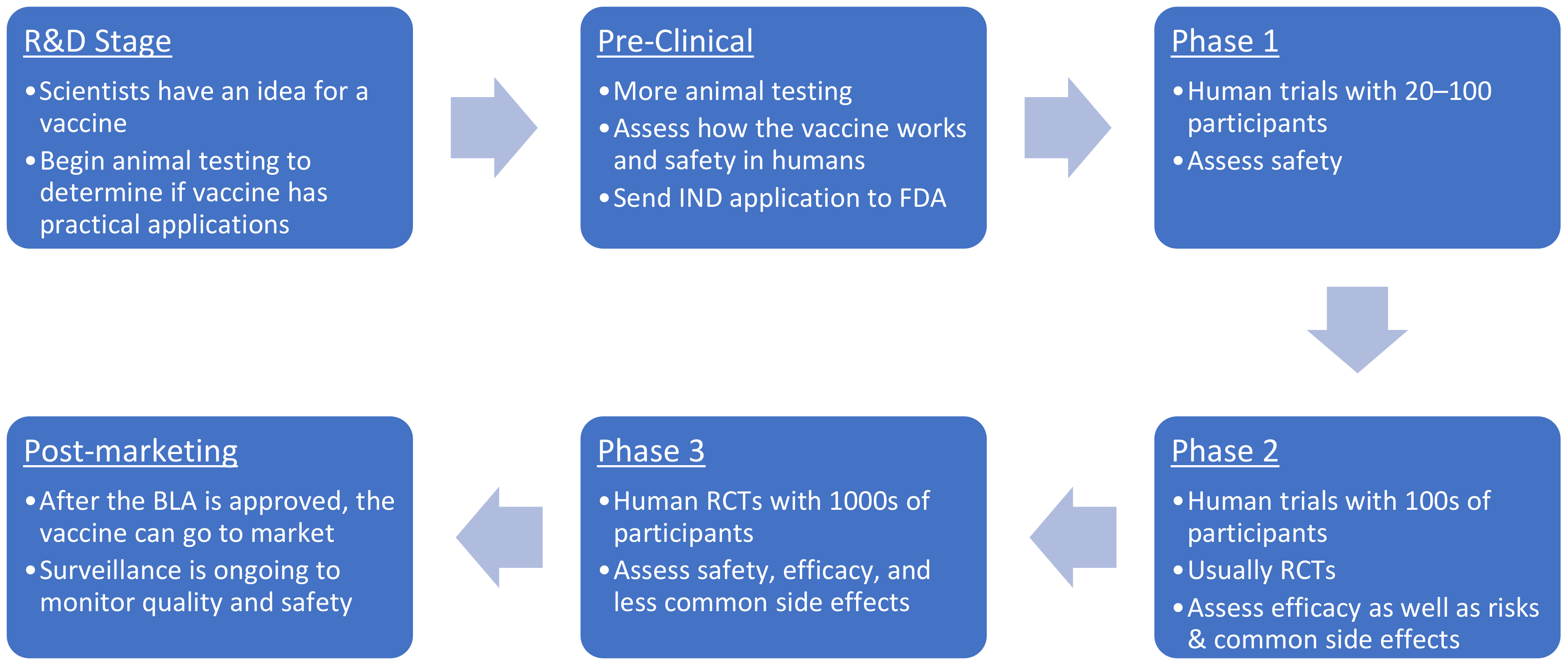
Vaccines, Free Full-Text
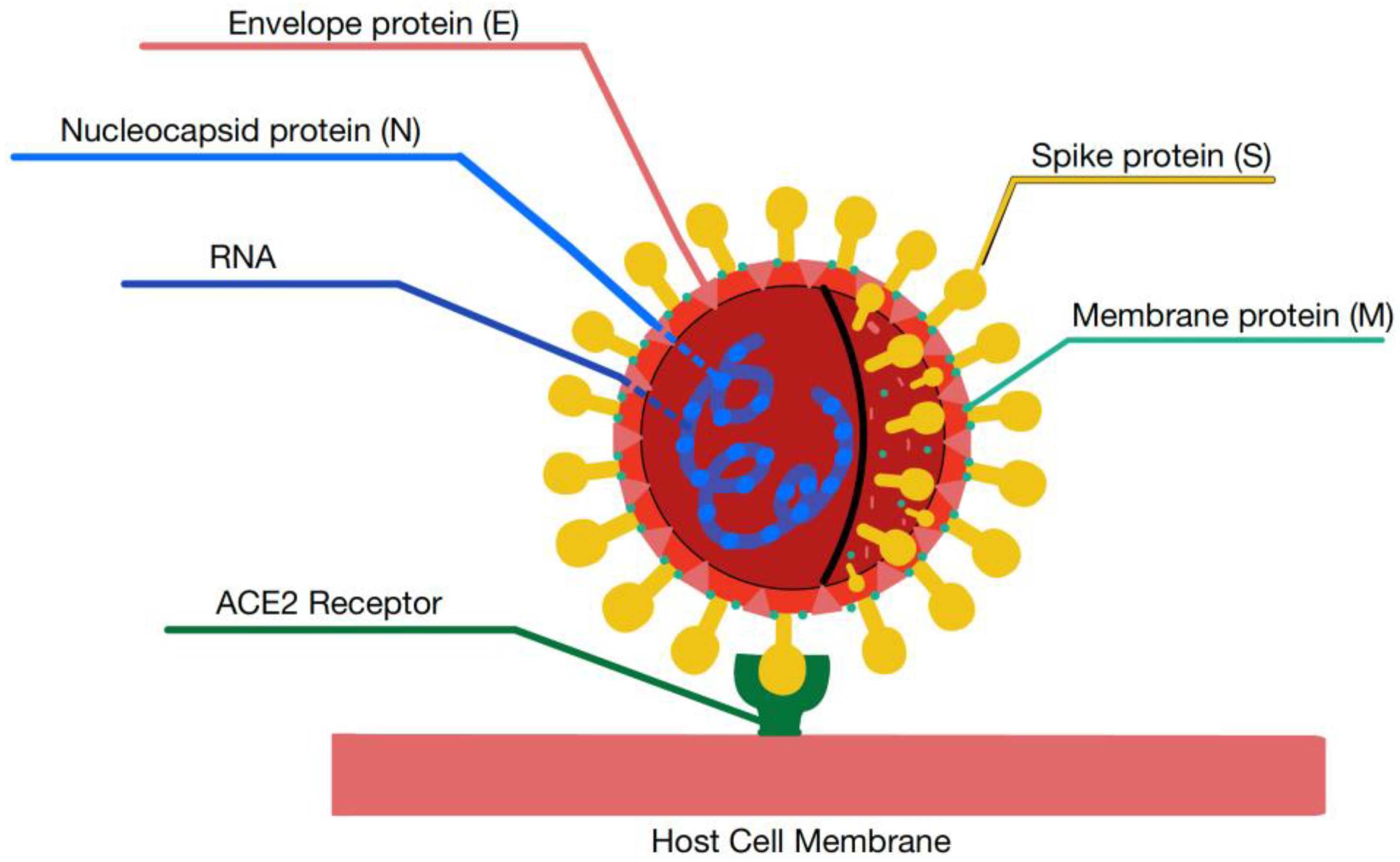
Biomolecules, Free Full-Text

Emergency Use Authorization

Coronavirus Drug and Treatment Tracker - The New York Times
Recomendado para você
-
 O brit milá do caçula do chef Isaac Azar03 junho 2024
O brit milá do caçula do chef Isaac Azar03 junho 2024 -
 Covid-19: Birx Lashes Trump's Pandemic Response and Says Deaths Could Have Been 'Decreased Substantially' - The New York Times03 junho 2024
Covid-19: Birx Lashes Trump's Pandemic Response and Says Deaths Could Have Been 'Decreased Substantially' - The New York Times03 junho 2024 -
 Isaac Azar, MD, Aventura, FL Emergency Medicine Physician03 junho 2024
Isaac Azar, MD, Aventura, FL Emergency Medicine Physician03 junho 2024 -
 Mahmoud Al-Hawary MD Anderson Cancer Center03 junho 2024
Mahmoud Al-Hawary MD Anderson Cancer Center03 junho 2024 -
 John van Salee de Grasse - Wikipedia03 junho 2024
John van Salee de Grasse - Wikipedia03 junho 2024 -
 Dr. Frederick Kaplan, M.D. - 2017 Rare Impact Award Honoree - National Organization for Rare Disorders03 junho 2024
Dr. Frederick Kaplan, M.D. - 2017 Rare Impact Award Honoree - National Organization for Rare Disorders03 junho 2024 -
 Dr. Rodriguez Lopez - Mexico Bariatric Center - Over 4,000 Surgeries03 junho 2024
Dr. Rodriguez Lopez - Mexico Bariatric Center - Over 4,000 Surgeries03 junho 2024 -
BUFFET COMPLETASSO COM OPEN DE HÄAGEN-DAZS E NUTELLA 😱😱 Fala Desbra03 junho 2024
-
 University News Royal News: December 16 202303 junho 2024
University News Royal News: December 16 202303 junho 2024 -
 Society Rio-SP, Gente que acontece! Negócios, Lifestyle, Festas e Carnaval03 junho 2024
Society Rio-SP, Gente que acontece! Negócios, Lifestyle, Festas e Carnaval03 junho 2024
você pode gostar
-
 Carlsen's Bizarre Decision Has Sent The World Chess Championship To Overtime03 junho 2024
Carlsen's Bizarre Decision Has Sent The World Chess Championship To Overtime03 junho 2024 -
 상대 벽느끼게 해줌03 junho 2024
상대 벽느끼게 해줌03 junho 2024 -
 No.2 Swiatek beats No.1 Sabalenka in WTA Finals semis - SportsDesk03 junho 2024
No.2 Swiatek beats No.1 Sabalenka in WTA Finals semis - SportsDesk03 junho 2024 -
![Hans Zimmer: Man of Steel Main Theme [Extended by Gilles Nuytens]](https://i.ytimg.com/vi/Nutm8Nwk5xM/maxresdefault.jpg) Hans Zimmer: Man of Steel Main Theme [Extended by Gilles Nuytens]03 junho 2024
Hans Zimmer: Man of Steel Main Theme [Extended by Gilles Nuytens]03 junho 2024 -
 Pablo Schreiber habla sobre su papel como Jefe Maestro en Halo y anuncia que en verano empezará a rodarse la temporada 203 junho 2024
Pablo Schreiber habla sobre su papel como Jefe Maestro en Halo y anuncia que en verano empezará a rodarse la temporada 203 junho 2024 -
 meme #memes #shrek #tpose #freetoedit - T Pose Shrek Png, Transparent Png , Transparent Png Image - PNGitem03 junho 2024
meme #memes #shrek #tpose #freetoedit - T Pose Shrek Png, Transparent Png , Transparent Png Image - PNGitem03 junho 2024 -
 Rainbow Friends Green Cutting File Cut File Cricut Plotter03 junho 2024
Rainbow Friends Green Cutting File Cut File Cricut Plotter03 junho 2024 -
 Baixar Car Parking Multiplayer Apk Mod Dinheiro Infinito03 junho 2024
Baixar Car Parking Multiplayer Apk Mod Dinheiro Infinito03 junho 2024 -
 Patch Pes 2017 MercadoLivre 📦03 junho 2024
Patch Pes 2017 MercadoLivre 📦03 junho 2024 -
 Alex Hogh Andersen - behind the fierce Ivar the Boneless03 junho 2024
Alex Hogh Andersen - behind the fierce Ivar the Boneless03 junho 2024
